GMP-compliant digitalization of maintenance in the pharmaceutical industry
Whether cleanroom, filling line or packaging unit – in pharmaceutical production, maintenance is a central pillar of product quality. Every measure must be planned, documented, approved and traceable – and remain verifiable at all times.
With SIMPL, you can manage your maintenance digitally and in compliance with GMP: record malfunctions, carry out maintenance, report back on measures – mobile, seamless and auditable. For smooth processes, less paperwork and more security during inspections by the authorities.

30
274.000
20
GMP requirements are increasing - but the processes remain analog?
In pharmaceutical production, maintenance is a key regulatory issue. Every maintenance, every inspection and every release must be fully documented, archived in a version-safe manner and auditable at any time. However, in many places these processes are still mapped using paper forms, Excel spreadsheets or decentralized systems.
This leads to high administrative costs, error-prone reworking and risks during audits. If maintenance is incompletely documented, measures are incorrectly assigned or deadlines are overlooked, not only does production come to a standstill – GMP compliance is also at stake.
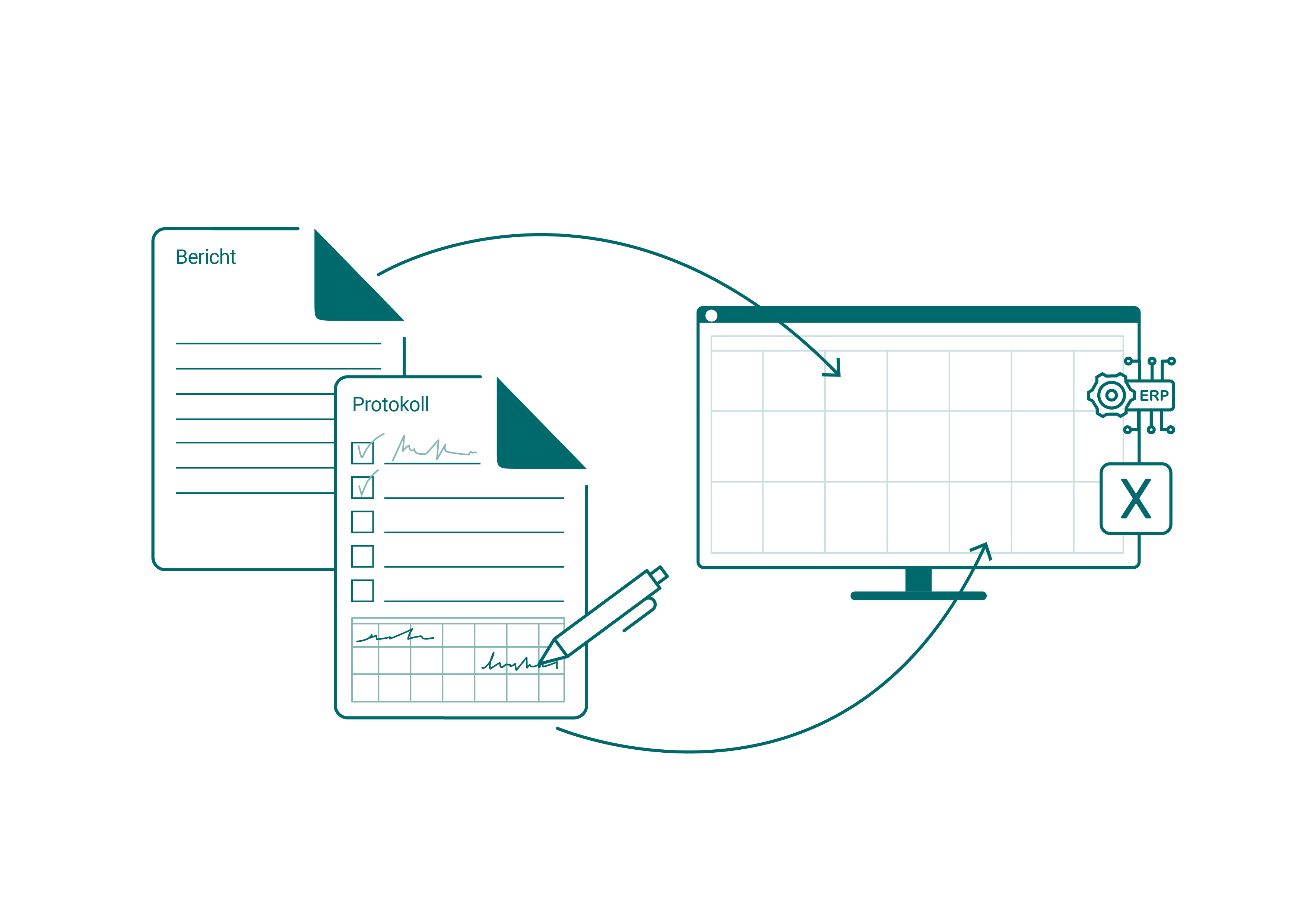
Organize GMP-compliant maintenance digitally - seamlessly and efficiently
With SIMPL, you can manage your maintenance completely digitally and in compliance with GMP. All measures – from fault reports and planned maintenance to final approval – are documented in a traceable manner, automatically assigned and stored centrally.
Checklists, deadlines, responsibilities and release notes can be accessed at any time – mobile, structured and auditable. You reduce paperwork, prevent errors in feedback and ensure that your maintenance is on safe ground from a regulatory perspective – even for audits at short notice.
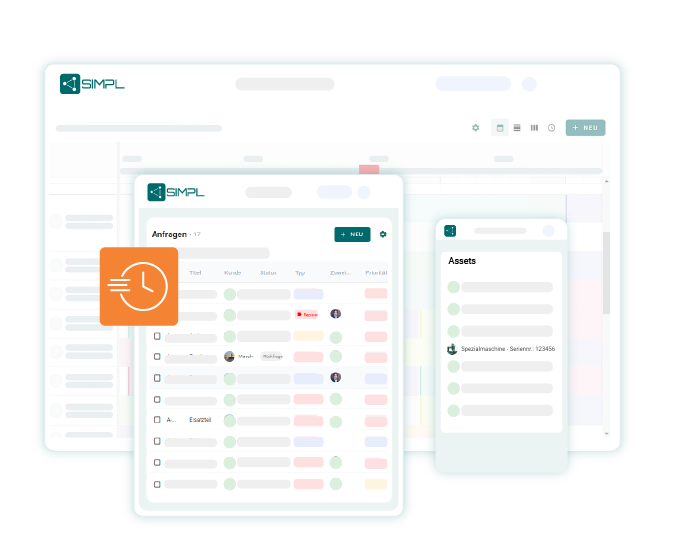
Control maintenance planning & inspection obligations in compliance with GMP
With SIMPL, you can plan recurring maintenance, inspections and calibrations in a structured way – including responsibilities, deadlines, GMP-relevant specifications and digital checklists. This allows you to keep track of critical systems – and never miss an approval again.
All test cycles, system approvals and measures safely under control
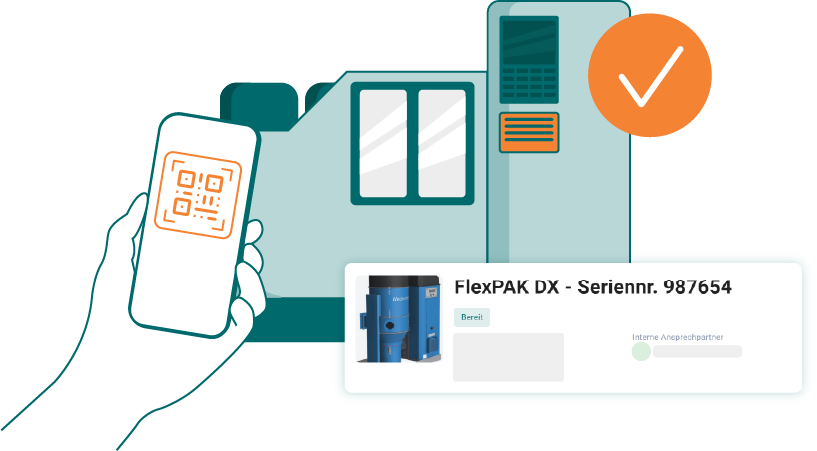

Mobile feedback with GMP release & audit trail
Technicians document all activities on the move – with a digital checklist, time stamp, signature and GMP-compliant release note. All data is stored in a version-safe manner and can be checked at any time – complete, structured and audit-proof.
Complete documentation of measures – directly at the plant
Evaluation & audit preparation at the touch of a button
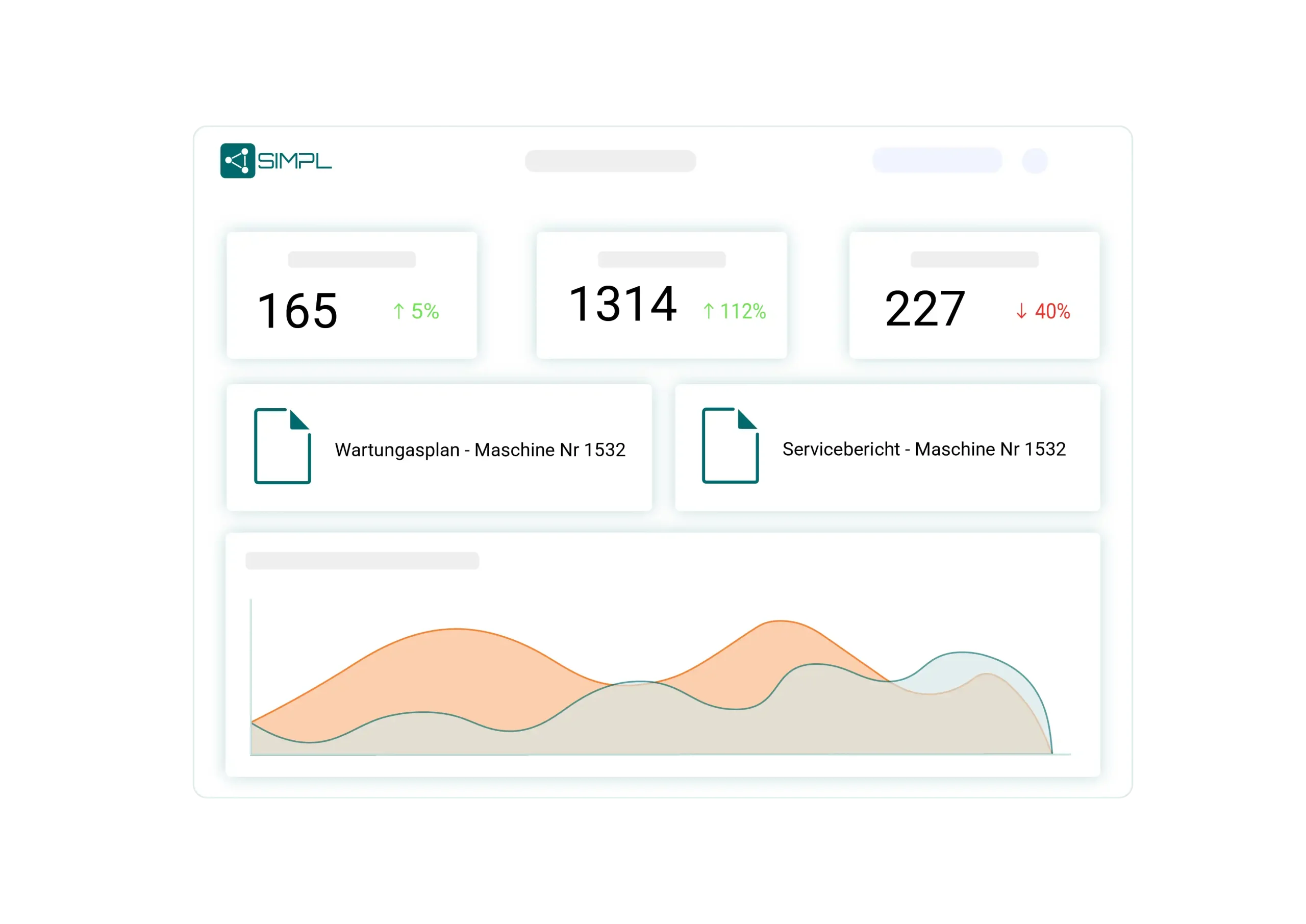
With SIMPL, you can analyze performed maintenance, overdue inspections or recurring faults in real time. You export reports for audits, authorities or internal quality management – quickly, standardized and legally compliant.
Measures, deadlines and inspection status can be displayed transparently at any time
Meet GMP requirements – with maintenance that thinks digitally
With SIMPL, you can manage your maintenance in a structured, traceable and auditable way – from planning to digital approval.
Individual support from real contacts
We accompany you personally – with support that understands your industry. No call center, no script – just fast, direct help from people who really know SIMPL.
Intuitively developed for use on site
SIMPL also works where time is of the essence: mobile, offline-capable, without long training periods. Your technicians will get to grips with it straight away – whether using a tablet or smartphone.
Made & hosted in Germany
Our servers are located exclusively in Germany, are ISO 27001-certified and GDPR-compliant. Do your customers require confidentiality? We provide it as standard.






